Every living thing moves—prey from predators, ants to crumbs, leaves toward sunlight. But at the most fundamental level, scientists are still struggling to grasp the physics behind how our own cells build, move, transport and divide.
“The mechanisms that allow organisms to move and change shape are inherent to life, and they are all underlaid by physics,” said Margaret Gardel, professor of physics at the University of Chicago. “But despite how central they are for our understanding of biology, a great deal of these remain poorly understood.”
Gardel led an innovative new study, which for the first time recreates the mechanism of cell division—outside a cell. The experiment, led by postdoctoral fellow Kim Weirich and published May 21 in the Proceedings of the National Academy of Sciences, helps scientists understand the physics by which cells carry out their everyday activities, and could one day lead to medical breakthroughs, ideas for new kinds of materials or even artificial cells.
“How cells divide is one of the most basic aspects of trying to create life, and it’s something we’ve been trying to understand for hundreds of years,” said study senior author Gardel, who combines physics and biology to study the ways by which cells transform themselves.
Cells move through the body, but some of the most complex motion takes place inside the cell, as it ships ingredients and supplies from place to place, flattens or expands, and divides to recreate itself. One of the key players in this dance is actin, a protein that assembles itself into rods and structures.
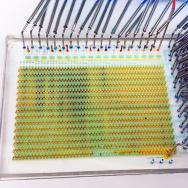
Gardel’s team wanted to understand the physics behind the actions of actin. So Weirich turned to one of the main ways that scientists have for this question: take the ingredients and try to build with them outside the cell.
Weirich separated out actin proteins, and watched as they formed droplets that took on an almond shape. When Weirich added myosin (“motor” proteins common in muscles), they spontaneously found the center between the two ends of the droplet and pinched off the droplet into two.
They were totally shocked to see the process, Gardel said. “There’s no precedent for this. It looks exactly like the spindles that drive cell division.”
Working with fellow UChicago physicist Thomas Witten and chemist Suriyanarayanan Vaikuntanathan, postdoctoral fellow Kinjal Disbaswas modeled the physics at play.
When in a droplet, the rod-like actin molecules like to align themselves in parallel to minimize conflict, forming the almond shape. The longer myosin molecules prefer to gather in the center so that they can still stay parallel to the actin. But as more myosins gather, they begin to stick together, forming clusters that favor tilting rather than staying parallel—so it pinches off into two. It’s the first such detailed look at how a cell might accomplish this task.
Watching this process—how living things exploit the structure of a droplet to form more life—is not only fascinating but useful, Gardel said. Though the types of proteins are different in cell division, the underlying principles are likely similar. “This is the kind of thing you need to know to imagine building things like artificial tissue for a wound,” she said.
“Ultimately, a great deal of problems in biology are about how ensembles of molecules work together,” she said, “and because these are often materials with chemical reactions going on inside, they’re very hard to model. These kinds of studies allow us the opportunity to explore the basic principles of the forces at play.”
Citation: “Self-organizing motors divide active liquid droplets.” Weinrich et al, PNAS, May 21, 2019. DOI: 10.1073/pnas.1814854116
Funding: National Science Foundation, Sloan Fellowship, National Institutes of Health.